What are Poison and Poisoning?
Poisons are substance that is assimilated through ingestion or inhalation or by any other contact which leads to the deterioration of normal functions of the body and eventually to death by its effects.
Poisoning is the process of intoxication by poisonous substances through ingestion or inhalation which causes detrimental effects on the functions of the body.
Toxicity defined as the amount of the substance (toxins and/or poisons) that can cause damage to bodily functions and leads to adverse effects.
Sources of Poison
Poisonous substances can be naturally occurring from plants and can also be synthetic substances like pesticides, insecticides, and even the overdose of the medication can also lead to poisoning effects on the body.
Hence, any substances can be a poison, if taken beyond its considerable limits.
But, here we are going to discuss only those which produce toxins and are harmful for human consumption. So, these are some of the common sources of poison that are usually encountered.
1. Domestic or Household sources: Common sources are detergents, disinfectants, cleaning agents, antiseptics, insecticides, rodenticides, etc.
2. Agricultural and Horticultural sources: Different insecticides, pesticides, fungicides, and weed killers.
3. Industrial sources: In factories, where poisons are manufactured or poisons are produced as by-products.
4. Commercial sources: From storehouses, distribution centers, and selling shops.
5. Drugs and medicines: Due to wrong medication, overmedication, and abuse of drugs.
6. Food and drink: Contamination in way of the use of preservatives of food grains or other food material such as additives (like coloring and odouring agents), or other ways of accidental contamination of food and drink.
7. Miscellaneous sources: Snakebite poisoning, city smoke, sewer gas poisoning, etc.
Classification of Poison
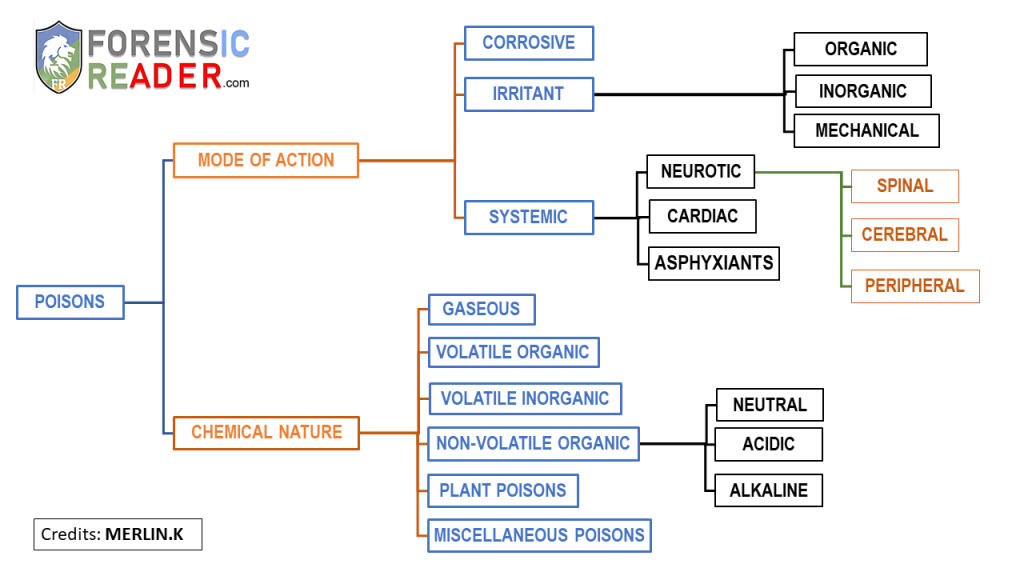
While classifying the poisons, there are mainly two characteristics to be considered.
Both of them have equal importance in analyzing and determining of type of action.
The two main characteristics are mode of action and chemical nature.
1. Based on Mode of Action
Poisons classified under the mode of action are collectively subdivided into corrosive, irritants, and systemic poisons.
The Irritant poisons grouped as organic, inorganic, and mechanical poisons. Systemic poisons are grouped into neurotic, cardiac and asphyxiated poisons. Moreover, neurotic poisons are further grouped into cerebral, spinal, and peripheral.
2. Based on Chemical Nature
According to chemical nature, the poison was grouped into gaseous, volatile organic, volatile inorganic, non-volatile organic poisons of neutral, acidic and alkaline, plant poisons, and miscellaneous poisons.
Poison Categorized Based on Mode of Action
Mode of action defined to be a modification that may be functional or anatomical from the cellular level, which is due to the exposure of poisons.
A. Corrosive Poisons
These poisons generally cause inflammation and ulceration of the tissues exposed.
Their mechanism involves extracting water from the tissues and which coagulate the cellular proteins and also converts the hemoglobin into haematin. The corrosive poisons can be strong acids and alkali.
Strong Acids: mineral acids, such as sulphuric acid, nitric acid, and hydrochloric acid; organic acids, such as oxalic acid, carbolic acid, acetic acid, salicylic acid.
Strong Alkali: caustic soda, carbonates, ammonium, sodium, and potassium.
B. Irritant Poisons
This group of poisons affects the abdomen indicated by vomiting and purging. They also cause inflammation and ulceration in the gastrointestinal tract. Generally, the diluted corrosives act as irritants.
Based on the compounds present on the poisons, they are further classified as:
1. Inorganic Irritants
This group consists of non-metallic and metallic poisons. Following are the example of inorganic irritants:
- Metallic: arsenic, antimony, mercury, lead, copper, thallium, zinc, manganese, barium, and radioactive substances
- Non-metallic: phosphorous, chlorine, bromine and iodine
2. Organic Irritants
This group comprises animal and plant poisons. For example:
- Plant poisons: castor, marking nut, ergot, calotropis, etc
- Animal poisons: snakes’ venom, insects, cantharides, and spiders.
3. Mechanical Irritants
Powdered glass, diamond dust and chopped hairs, dried sponges, etc.
C. Systematic Poisons
This group of poisons affects the vital systems of the body. It generally affects the nervous system, cardiovascular system, and respiratory system.
Based on their activity on the respective systems, they are classified as Neurotic poison, Cardiac poison, and Asphyxiant poisons.
1. Neurotics Poisons
These poisons act primarily on the nervous system through local irritant action. Majorly, alkaloids are included in this group.
a. Cerebral poisons: If a neurotic poison acts majorly on the cerebral parts, especially on the cerebrum, they are called cerebral poisons. Examples are:
- somniferous such as opioids.
- Inebriants such as alcohol, anesthetics, ether, sedatives and hypnotics, fuels, and agrochemical compounds
- Deliriants, such as dhatura, cannabis, cocaine, belladonna
b. Spinal Poisons: if the poisons act on the spinal part, it is termed as spinal poisons. Example: Nux vomica and its alkaloids, gelsemium.
c. Peripheral Poisons: Poisons acting on the peripheral nerves are called as peripheral poisons. Example: curare and conium
2. Cardiac Poisons
These are poisons that act on the heart either by direct effects or by affecting the musculature which further responsible for the damage in nerve supply.
Example: digitalis, oleander, aconite, and nicotine.
3. Asphyxiant Poisons
These poisons acts on the respiratory system by affecting oxygen intake and also cause the depletion of oxygen.
Example: carbon monoxide, carbon dioxide, sewer gases and some war gases.
Poison Categorized Based on Chemical Nature
In the toxicological analysis, it is required to know about chemical properties and its isolation methods. Hence, the poisons are classified on their chemical nature.
1. Gaseous Poisons
These types of poisons are inhaled and directly affect the oxygen carrier i.e. blood, and further damages the tissues of the air passages and lungs.
Examples: carbon monoxide, carbon dioxide, hydrogen sulphide, sulphur oxide, chlorine, nitrous oxide, tear gas, etc.
2. Volatile Inorganic Poisons
This group of substances usually follows the slow inhalation of vapors in order to become intoxicated.
Example: Cyanide, phosphine, arsine, phosgene, chloride, etc.
3. Volatile Organic Poisons
These substances have high vapor pressure at ordinary room temperature. The high vapor pressure was a result of a low boiling point, which leads to the sublimation of large numbers of molecules from the liquid or solid form of the compound and enters the surrounding air.
Examples: ethanol, ethanol, formaldehyde, and acetaldehyde.
4. Non-Volatile Inorganic (Anions) Poisons
These include compounds that possess the anionic property
Examples: anionic halides, dichromate, chlorates, azide, nitrites, sulphate, phosphide, cyanide etc.
5. Non-Volatile Inorganic (Cations) poisons
These include a compound that has cationic properties.
Example: mercury, arsenic, barium, thallium, lead, antimony, bismuth, etc.
6. Non-Volatile Organic Neutral Poisons (Pesticides)
The common constituents of pesticides are grouped under this.
Examples: organophosphates, organochlorates, carbamates, and pyrethroids.
7. Non-Volatile Organic Acidic Compound (Acidic Drugs)
The substances that are acidic in nature, are called Acidic Drugs. These drugs readily react with bases to form salts.
Examples: barbiturates, sulpha, phenolic compounds (Phenol, Cresols, etc.), and salicylates.
8. Non-Volatile Organic Alkaline Compounds (Basic Drugs)
When the substance contains a nitrogen atom with a lone pair of electrons available for reaction with protons. Therefore, they will behave as bases and are grouped under alkaline compounds.
Examples: alkaloids, benzodiazepines.
9. Plant Poisons
The active constituents (or principles) of plants that cause toxic effects which is organic compounds that are non-volatile in nature.
Examples: Dhatura, aconite, oleander, nux vomica, etc.
10. Miscellaneous Poisons
These poisons may be organic or inorganic, volatile or non-volatile, and (or) animal or plant origin, or toxins produced thereof.
- Mechanical poisons: diamond dust, glass powder, and chopped hair.
- Food poisoning: mycotoxins, bacteria, and food-borne microbes.
- Animal /insect poisons: snake venom, scorpion, poisons bees, poisonous ants.
Types of Poisoning

Poisoning can be defined as the process that takes place after the intoxicated action of a substance. The action may be homicidal, suicidal, or accidental. Majorly, poisoning can be classified based upon their exposure.
A. Acute Poisoning
It is caused by an excessive single dose (highly toxic substances having a small fatal dose), or several doses of a poison taken over a short interval of time.
Symptoms of Acute Poisoning
The following groups of symptoms are suggestive of acute poisoning:
- Sudden onset of abdomen pain, nausea, vomiting diarrhea, and collapse.
- The sudden onset of coma with constriction of pupils.
- The sudden onset of convulsion.
- Delirium with dilated pupils.
- Paralysis, especially of lower motor neuron type.
- Proteinuria and hematuria.
- Persistent cyanosis.
- Rapid onset of neurological or gastrointestinal illness in persons known to be occupationally exposed to chemicals.
B. Chronic Poisoning
It is caused by smaller doses over a long period of time, resulting in gradual worsening. The poisons which are commonly used for the purpose of chronic poisoning are arsenic, antimony, phosphorous, and opium.
Symptoms of Chronic Poisoning
- The symptoms are exaggerated after the administration of suspected food, fluid, or medicine.
- Depression and gradual deterioration of the general condition of the patient are seen.
- Repeated attacks of diarrhea, vomiting, etc. are seen.
- When the patient is removed from his usual surroundings, the symptoms disappear.
- Traces of poison may be found in the urine, stool, or vomit.
C. Subacute Poisoning
In sub-acute poisoning, the exposure level is lower and the survival time longer, than in acute poisoning, but the period between exposure and manifestation of signs of poisoning and possible death is, again, relatively short. Symptoms of toxicity develop gradually with time.
D. Fulminant Poisoning
It is produced by a massive dose. In this death occurs suddenly, sometimes without showing any symptoms.
Sample Collection in Case of Poisoning
During the analysis, when the victim is suspected to be poisoned then there are certain measures to be taken in the crime scene such as searching for the poisonous substance and collecting the required sample from the victim in fatal cases and also in survival cases.
A. Sample Collection in Survival Cases
Following materials should be collected for the detection of poison from the victim in survival cases, includes:
1. Stomach Wash
2. Vomit
3. Blood (10mL). For 10 mL of blood, 100 mg of sodium fluoride is added, which acts as both a preservative and as an anticoagulant.
4. Urine, as much as possible.
5. Feces Materials
6. Suspected Materials recovered from the CS. (E.g. Empty medicine vial remains of food or drinks, Residual poison, strips of
tablets and capsules, etc.)
B. Sample Collection in Fatal Cases
Appropriate materials for the detection of poison from the victim in fatal cases include:
1. All the materials collected in survival cases (Stomach Wash, Vomit, Blood, Urine, Feces Materials),
2. The stomach and its content,
3. Small intestine and its contents
4. Liver and gall bladder
5. Half of each kidney
6. Spleen
7. Lungs
8. Heart
9. Limb tissue (In case of snakebite)
10. Uterus with fetus (In case of criminal abortion)
11. Hair with roots, nails, long bones (in case of suspected chronic metabolic poisoning)
12. Skin from the site of injection or bite along with a control skin from other sites of the body.
Techniques For Sample Collection in Poisoning Cases
1. Blood: Blood samples are best obtained from the femoral artery or vein by percutaneous puncture using a 50 ml syringe with a wide-bore needle. Try to collect blood samples of about 50 ml of blood but should be at least 10 ml. Sample should be preserved with sodium fluoride/ potassium oxalate unless suspicion of poisoning with fluoride.
2. Urine: Urine in dead may be collected by direct puncture with a needle and syringe of the exposed bladder after the abdomen has been opened. It may also be obtained by insertion of a urethral catheter before starting the postmortem. The use of fluoride as a preservative is encouraged.
3. Cerebro-spinal Fluid: It should be collected by cisterna puncture or aspirating with a pasture pipette from the base after the frontal lobes of the skull has been opened. The maximum possible amount should be withdrawn.
4. Bone and Bone Marrow: 8-10 cms portion of the shaft of the femur should be taken. The required amount of bone marrow should be withdrawn from the sternum or femur.
5. Hair: About 10 gm or less, if available. The head and public hair should be plucked out along with the root, and not by shaving. The best collection site for head hair is from the vertex posteriorly on the back of the head.
6. Muscles: About 10gm wedges of thigh or chest muscles are collected before the abdomen is opened. 100-200 mg of the muscles can be the ideal tissue for DNA extraction.
7. Nails: All the nails (from fingers and toes) should be removed entirely and collected in a separate clean envelope.
8. Skin: A piece of at least 2.5cm square from the affected area (in case of corrosives or skin applications) and from thigh or back (in suspected metal poisoning). If there is a puncture like injection or animal bite, the whole needle track or bite mark along with the surrounding tissue with 5cm square skin should be excised. Control specimen should be taken from the opposite side of the body and preserved separately as control.
9. Bile: It is aspirated by a needle after the abdomen is opened and before the organs are removed. All available bile should be removed from the gall bladder and preserved with sodium fluoride. To avoid fermentation of this specimen, it should be stored at a temperature of at least -20°C.
10. Gastric Contents: All of the available gastric contents are collected without the addition of preservatives. Undigested pills and tablets should be separated and placed into plastic pillboxes for analysis.
11. Liver: Specimens from the right lobe are preferred from the left lobe to avoid spuriously high concentration from diffusion from the stomach.
12. Vitreous Humor: 2-3 ml of vitreous from one or both eyes is aspirated from the lateral angle of the eye with a 5ml syringe.
13. Lungs: In cases of inhalation and intravenous exposure the apex of either lung (25-50g) is the best choice of the specimen.
14. Kidney: A minimum of 25g sample of the kidney is recommended.
15. Stains: Blood, saliva, vomit, feces and urine stains on clothes, bedsheets and other cloth materials need to be air-dried before packing each article separately. The whole item or separate cut-outs of the stains can be packed. While sending the stained clothes, the surrounding unstained portion should also be collected as a control sample.
16. Remains of food and drink: Should be sent to the laboratory separately with or without preservatives as per their conditions.
Preservation of Collected Samples
Following are some of the pointers that you should be considered while preserving the samples for lab tests.
- Containers for the preservation of material should have a wide-mouth glass or plastic bottle of about 2L capacity along with an air-tight stopper. They majorly used for collecting visceral tissues or other biological samples.
- To collect liquid samples, the best containers would be hard disposable plastic or glass tubes.
- Containers should be numbered and labeled properly along with details such as the date of postmortem material obtained, added preservatives, and the signature of the postmortem officer.
Table For Collection & Preservation of Samples in Poisoning Cases
The following table assimilates the different cases and the preservatives that should be used during the collection of samples.
| Case History | Samples to be Collected | Preservative to be used |
| Normal Cases (Sp1) | Viscera (stomach, intestine along with contents) | Common salt or its saturated solution |
| Normal Cases (Sp2) | Blood (10-15cc.) | Potassium oxalate+ Sodium Fluoride |
| Acid poisoning (Sp1) | 1.Viscera (stomach, intestine along with contents) 2.liver, spleen, kidney | Rectified spirit |
| Acid poisoning (Sp2) | Blood (10-15cc.) | Potassium oxalate+ Sodium Fluoride |
| Cyanide poisoning (Sp1) | 1.Viscera (stomach, intestine along with contents) 2.liver, spleen, kidney | Common salt or its saturated solution |
| Cyanide poisoning (Sp2) | Blood (10-15cc.) | Potassium oxalate+ Sodium Fluoride |
| Carbon monoxide & (other gaseous poisonings) (Sp1) | Blood (10-15cc.) | Potassium oxalate+ Sodium Fluoride with paraffin |
| Carbon monoxide & (other gaseous poisonings (Sp2) | Lungs | Common salt or its saturated solution |
| Arsenic poisoning (Sp1) | 1.Viscera (stomach, intestine along with contents) 2.liver, spleen, kidney 3.Nails, Bones, Hair | Common salt or its saturated solution |
| Arsenic poisoning (Sp2) | Blood (10-15cc.) | Potassium oxalate+ Sodium Fluoride |
| Snakebite and injection cases (Sp1) | Blood (10-15cc.) | Potassium oxalate+ Sodium Fluoride |
| Snake bite and injection cases (Sp2) | Skin piece at the site of injection | Common salt or its saturated solution |
*Sp = Sample
References
- Trestrail, J. H. (2000). Types of Poisons. Criminal Poisoning, 27–44. doi:10.1007/978-1-59259-023-0_2
- Forensic Toxicology [ForensicIndia.com]
- Types of Poisons, Criminal Poisoning [Springer.com]
- The Chemistry of Poisons [Chem.fsu.edu]
- Forensic Toxicology by Robert WENNIG [Eolss.net]
- Toxicology Introduction [Bmcsagar.edu.in]
- Corrosive Poisoning [Link]

She is a research scholar plus a teaching assistant with expertise in Forensic biology, toxicology, and pharmacology subjects from Karunya Institute of Technology and Sciences (Deemed University), Tamil Nadu. She obtained her postgraduate degree in Human Genetics and Molecular biology. [Know More]

FR Author Group at ForensicReader is a team of Forensic experts and scholars having B.Sc, M.Sc, or Doctorate( Ph.D.) degrees in Forensic Science. We published on topics on fingerprints, questioned documents, forensic medicine, toxicology, physical evidence, and related case studies. Know More.
